51 | Add to Reading ListSource URL: www.tegramedical.comLanguage: English - Date: 2016-05-23 12:17:23
|
|---|
52 | Add to Reading ListSource URL: www.pmda.go.jpLanguage: English - Date: 2016-08-04 23:25:57
|
|---|
53 | Add to Reading ListSource URL: www.jetro.go.jpLanguage: English - Date: 2015-03-30 22:38:04
|
|---|
54 | Add to Reading ListSource URL: www.fda.govLanguage: English |
|---|
55 | Add to Reading ListSource URL: www.bizint.comLanguage: English - Date: 2016-04-11 20:57:04
|
|---|
56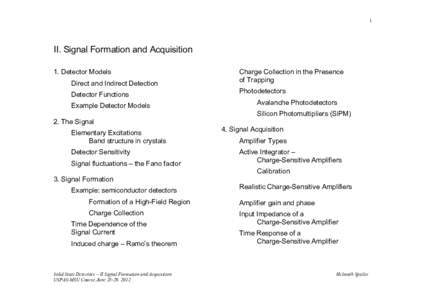 | Add to Reading ListSource URL: www-physics.lbl.govLanguage: English - Date: 2012-06-20 19:15:41
|
|---|
57 | Add to Reading ListSource URL: team.uhsystem.comLanguage: English - Date: 2016-01-07 12:08:43
|
|---|
58 | Add to Reading ListSource URL: www.auanet.orgLanguage: English - Date: 2016-08-17 17:13:12
|
|---|
59 | Add to Reading ListSource URL: www.ucrh.edu.auLanguage: English - Date: 2016-03-07 23:14:43
|
|---|
60 | Add to Reading ListSource URL: lasr.cs.ucla.eduLanguage: English - Date: 2012-09-17 16:56:34
|
|---|